Monoclonal antibodies (mAbs) represent an important and growing class of biotechnology-derived therapeutics. The mAb market is predicted by Grand View Research to reach USD 138.6 billion by 2024. One powerful application of Nidus technology is for high-throughput screening of antibody-secreting cells (ASC) to rapidly discovery mAb candidates.
Nidus technology is highly advantaged
- Overcomes limitations of conventional screening methods that are complex and labor intensive.
- Combines both limiting dilution and ELISA assays on-chip.
- Can screen mAb by epitope binding AND by function in situ.
- Functional screening confers a competitive edge providing customer value as studies show a poor correlation exists between mAb binding affinity and functional potency.
- Screening assays based on epitope binding, neutralization, phagocytosis, complement activation, and promoting cellular cytotoxicity are operational or in development. Contact us for more details.
EXAMPLE applications
In this type of application cells are seeded at low concentration into large arrays with smaller MB features. Cell seeding follows a Poisson distribution so the concentration can be adjusted to maximize the number of MBs that contain single cells. The image shows the 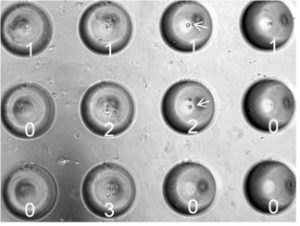 feasibility of this approach for seeding cell seeding, specifically each of the 12 MBs contain 0 to 3 cells. For more details on this data see Jones et al., “Characterization of cell seeding and specific capture of B cells in microbubble well arrays” Biomedical microdevices. 2013 Jun; 15(3):453-63.
feasibility of this approach for seeding cell seeding, specifically each of the 12 MBs contain 0 to 3 cells. For more details on this data see Jones et al., “Characterization of cell seeding and specific capture of B cells in microbubble well arrays” Biomedical microdevices. 2013 Jun; 15(3):453-63.
For some applications the presence or absence of cells growing or clonal morphology are productive endpoints; however, for antibody discovery the goal is to identify individual MBs in the array that contain cells producing antibodies that can bind a particular antigen or have a functional readout like neutralization or opsonization. Nidus has developed several screening assays based on both binding and function. The ability to culture cells in the the arrays for 1-3 days or longer enables the development of innovative functional screening assays that greatly enhances the efficient and value of the candidate ASCs discovered.
 This illustration shows an eexample of an epitope-based screening assay where secreted anti-tetanus toxin immunoglobulins from SA13 hybridoma cells were detected by a fluor-based reporter. For more details see, Bobo et al. “Microbubble array diffusion assay for the detection of cell secreted factors” Lab on a chip. 2014 Sep 21; 14(18):3640-50
This illustration shows an eexample of an epitope-based screening assay where secreted anti-tetanus toxin immunoglobulins from SA13 hybridoma cells were detected by a fluor-based reporter. For more details see, Bobo et al. “Microbubble array diffusion assay for the detection of cell secreted factors” Lab on a chip. 2014 Sep 21; 14(18):3640-50
Typical data obtained from a binding assay screen is shown in the below figure. The MBA™ is imaged using fluorescence (A) and in bright-field (B) of the same section of the array; the white arrows 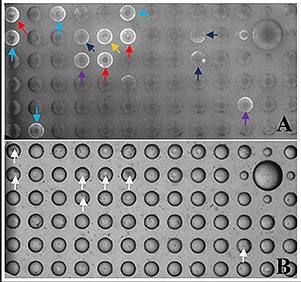 in (B) indicate MB wells with more than 8 cells. The bright fluorescent ring signals (A) indicate MBs with strong IgG secretion from SA13 cells, but also the ability to detect different levels of IgG production from these cells as indicated but the differing brightness of the fluorescent ring signals. Cells are recovered from individual MB wells using micro-manipulation tools for further characterization and work up e.g, Ig PCR amplification, gel analysis, cloning, scale up and validation.
in (B) indicate MB wells with more than 8 cells. The bright fluorescent ring signals (A) indicate MBs with strong IgG secretion from SA13 cells, but also the ability to detect different levels of IgG production from these cells as indicated but the differing brightness of the fluorescent ring signals. Cells are recovered from individual MB wells using micro-manipulation tools for further characterization and work up e.g, Ig PCR amplification, gel analysis, cloning, scale up and validation.
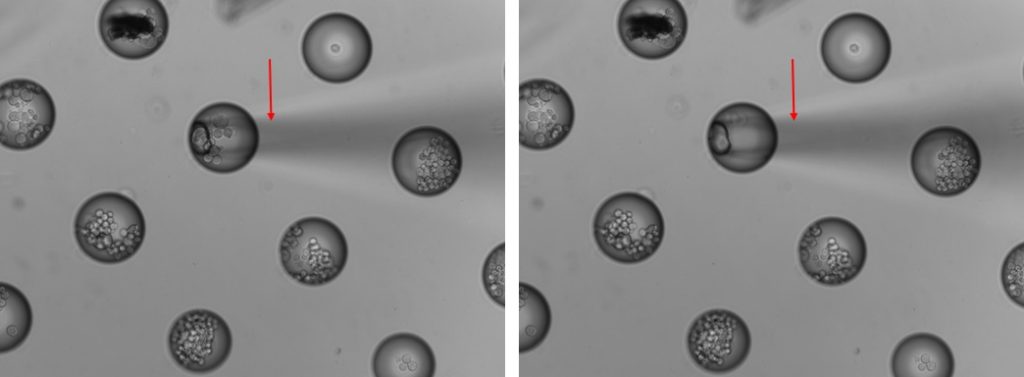
cell recovery from MB using micro-manipulation tools
validation of MBA™ For Antibody Discovery From Primary B Cells
MBA™ technology has been validate for use with primary (human and mouse) B cells. The fluorescence image below from the Bobo publication cited above shows that IgG secretion (white rings) can be detected from human primary B cells cultured in an MBA™ for 48 hours.


The image to the left shows a 10X image of three MB wells with live human B cells cells labeled in green and secreted IgG in red.